
Applying Hess's Law of Constant Heat Summation YouTube
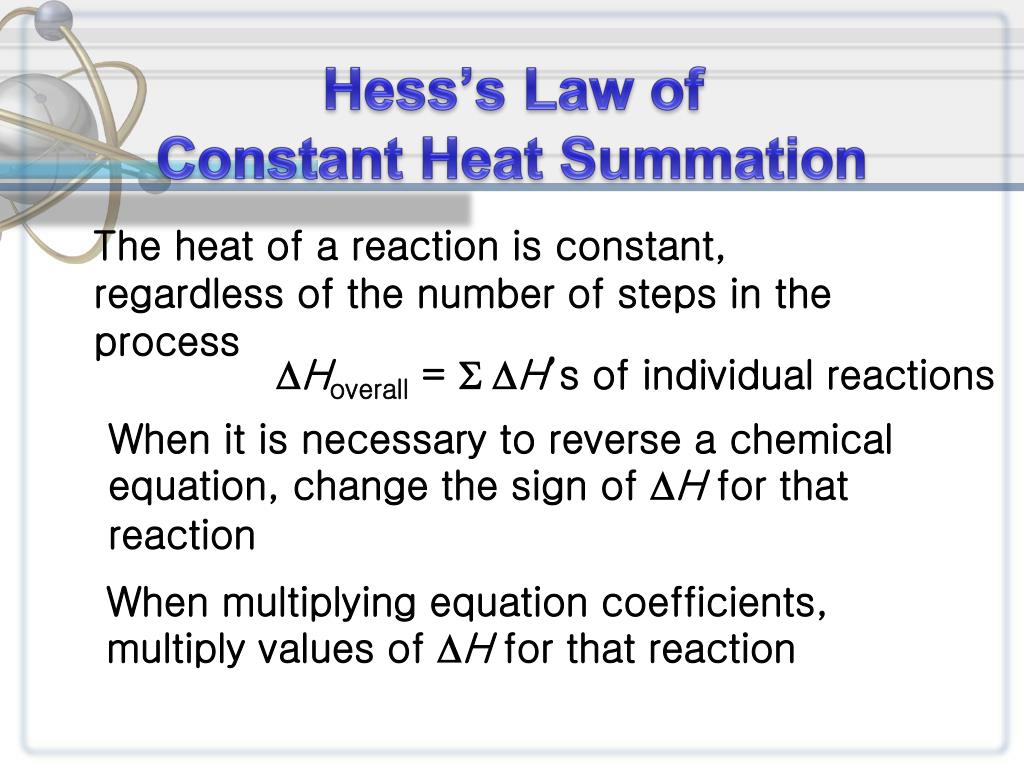
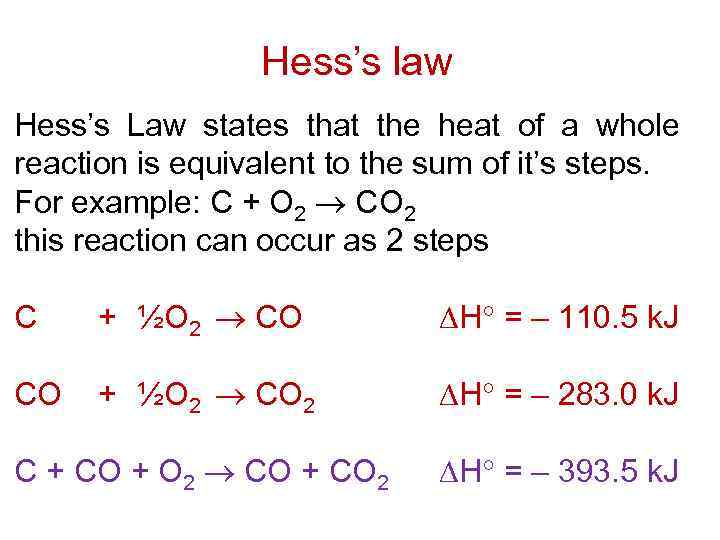
This type of calculation usually involves the use of Hess's law, which states: If a process can be written as the sum of several stepwise processes, the enthalpy change of the total process equals the sum of the enthalpy changes of the various steps.

PPT Energetics PowerPoint Presentation ID1204656
Therefore, the definition of Hess law of constant heat summation is: "The enthalpy change in a chemical reaction is the same and independent of the path or steps the reaction takes." Explain Hess's Law with Example Let us take an example to understand the Hess law of constant heat summation. Example 1
PPT Energetics PowerPoint Presentation, free download ID836262
Energy (enthalpy) of a system (molecule) is a state function. So, enthalpy of reactant and product molecules is a constant and does not change with origin and path of formation. The first law of thermodynamics states that the total energy of the substances before and after any (physical or chemical) change should be equal.
chemistry Hess’s Law of Constant Heat Summation
The law states that: ' The total amount of heat evolved or absorbed in a chemical reaction is the same, irrespective of whether the chemical reaction is taking place in one step or multiple steps.

Hess's Law of Constant Heat Summation Thermodynamics NCERT
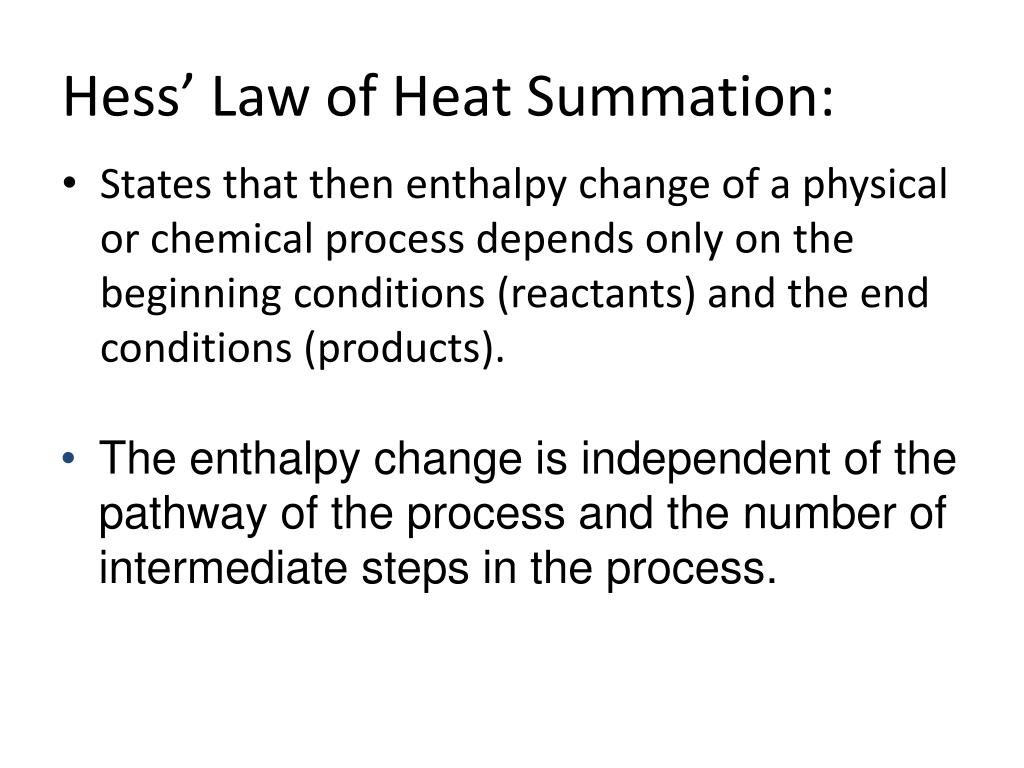
Hess's law, also called Hess law of constant heat summation, is one of the important outcomes of the first law of thermodynamics. The enthalpy change in a chemical or physical process is similar whether it is carried out in one step or in several steps.
PPT Hess’ Law of Heat Summation PowerPoint Presentation, free
The purpose of Hess's law is to measure the enthalpies of neutralization for several acid-base reactions, then use that information and Hess's law to determine the reaction enthalpies for two salts in aqueous solution. Table of Contents Application of Hess's Law Determination of Enthalpy of Formation Calculation of Standard Enthalpies of Reaction

Hess's Law of Constant Heat Summation, Chemistry Lecture Sabaq.pk
Additionally known as the Hess law of constant heat summation, is one of the key consequences of the first law of thermodynamics. Whether a chemical or physical process is carried out in one step or several steps, the enthalpy change is the same in both cases.

Hess's Law and Its Applications Hess's Law of Constant Heat Summation
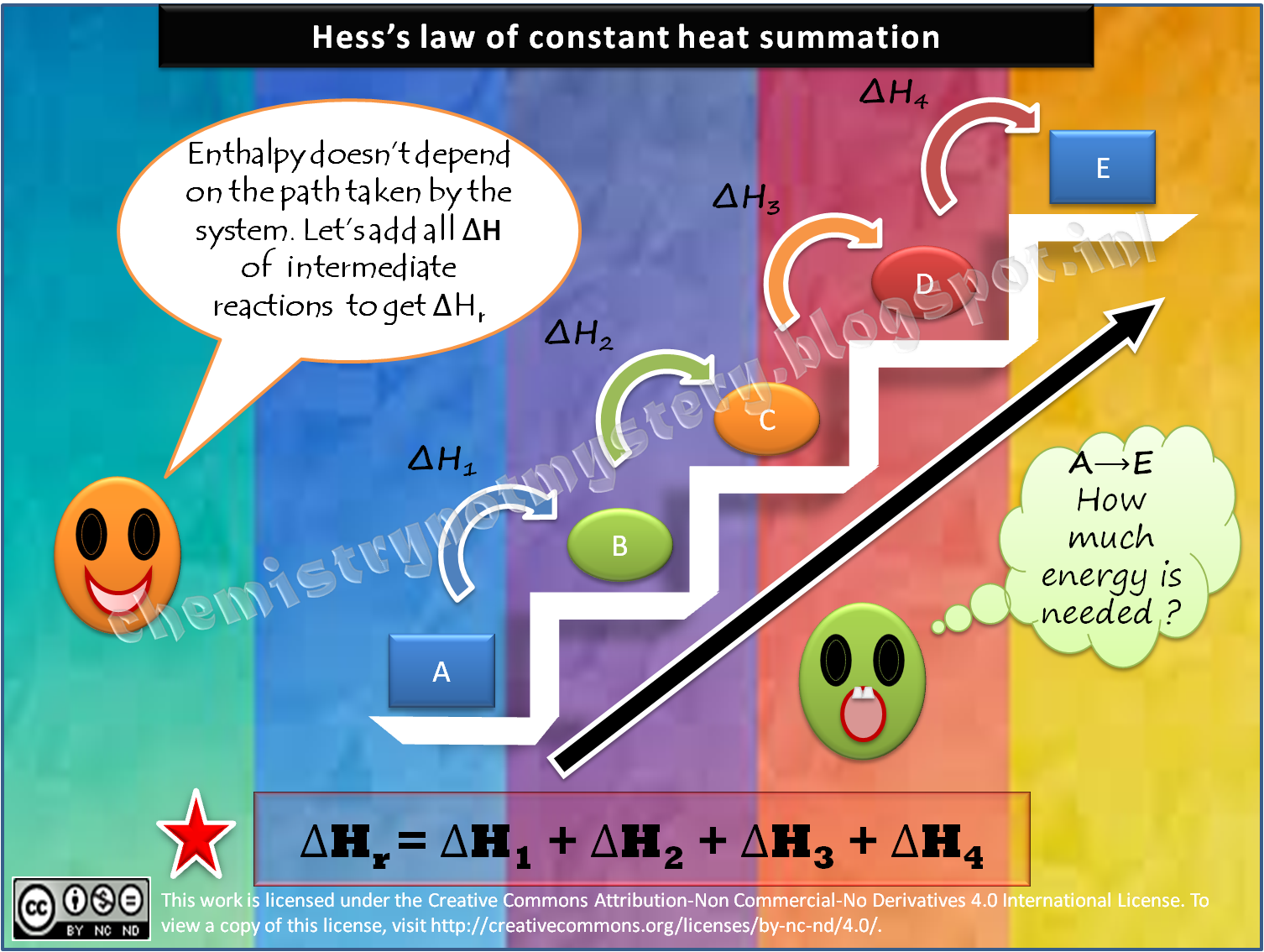
Hess's law of heat summation states that the total enthalpy change during a reaction is the same whether the reaction takes place in one step or in several steps. For example, in the above diagram, ΔH 1 = ΔH 2 + ΔH 3 = ΔH 4 + ΔH 5 +ΔH 6. In Hess's Law calculations, you write equations to make unwanted substances cancel out.
Hess Law chemistryatdulwich
17: Thermochemistry
Chemical thermodynamics lesson plan 1 Types of
Introduction. Definition: Hess's Law; Application; Why it works. Example 1; Contributors and Attributions; Hess's Law of Constant Heat Summation (or just Hess's Law) states that regardless of the multiple stages or steps of a reaction, the total enthalpy change for the reaction is the sum of all changes.This law is a manifestation that enthalpy is a state function.

PPT Hess’s Law PowerPoint Presentation, free download ID6193634
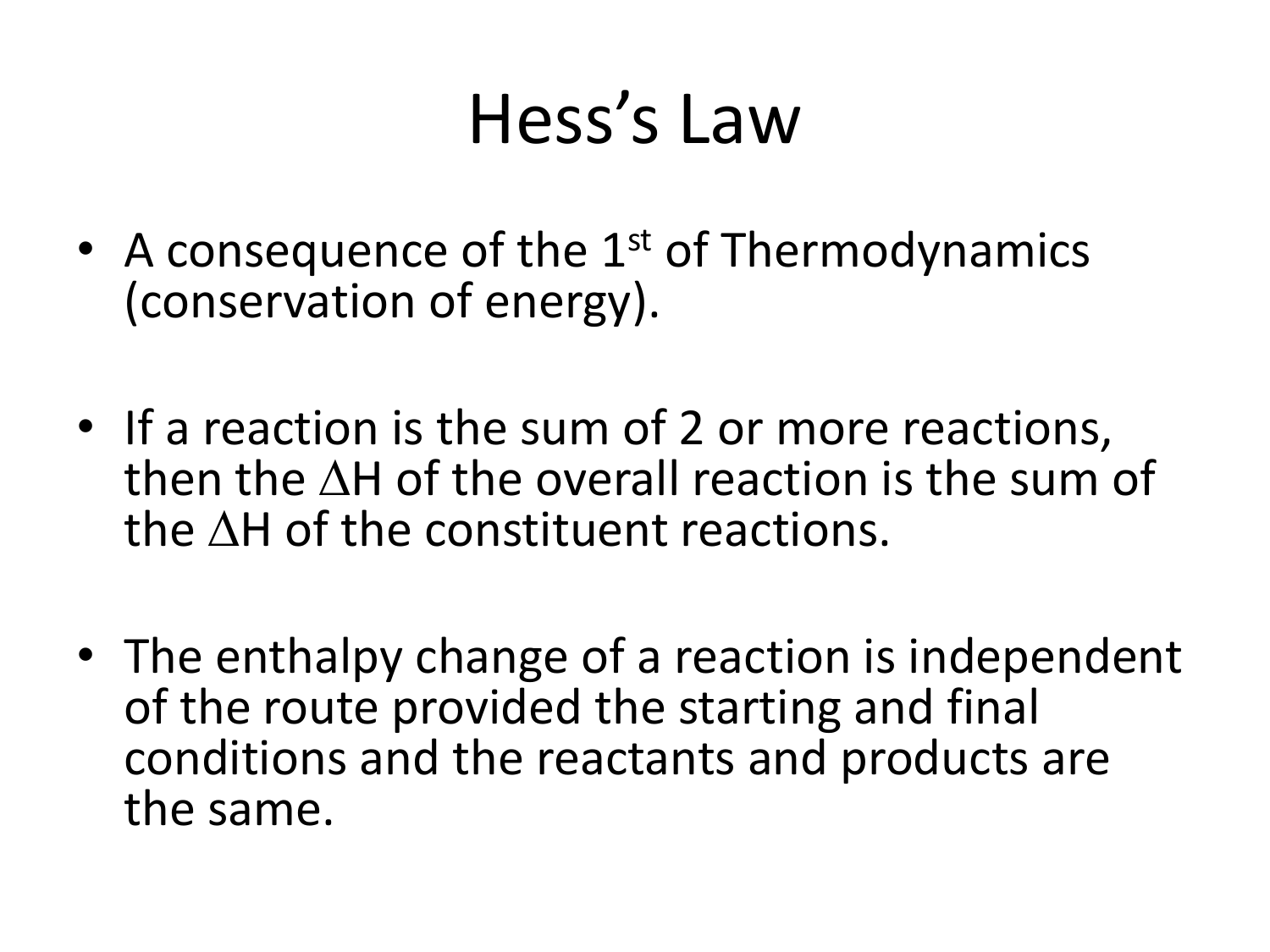
Hess's law, rule proposed by Germain Henri Hess, stating that the heat absorbed or evolved (or the change in enthalpy) in any chemical reaction is a fixed quantity and is independent of the path of the reaction or the number of steps taken to obtain the reaction.
Chem 1045 Lab hesss_law
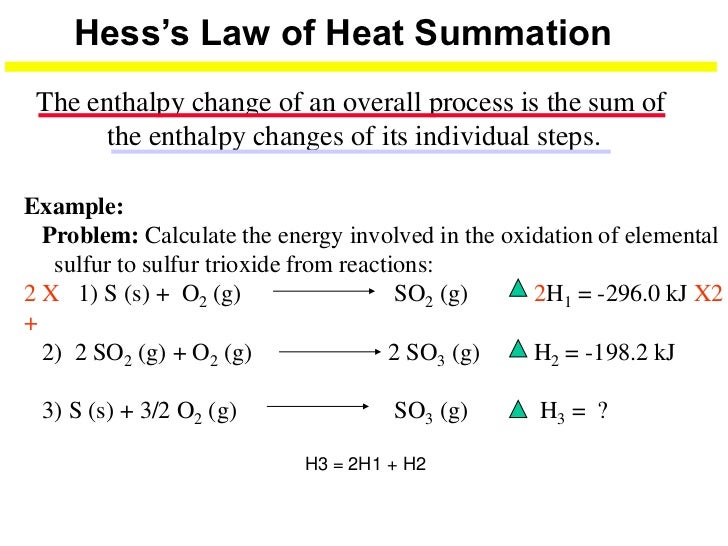
Applications of Hess's law of constant heat summation. This law can be used to determine the heat of the formation of a substance that cannot be measured experimentally. For example, the formation of benzene can not be prepared by combining the individual atoms such as carbon and hydrogen.

Chapter 09 20 Hess's Law of Heat Summation (graphically) YouTube
Hess's Law of Constant Heat Summation states that r, the total enthalpy change for the reaction is the sum of all changes and does not depend whether it takes place in single or multiple steps. This law is aan outcome of the fact that enthalpy is a state function. The applications of Hess's law are in . Calculation of enthalpy of formation

Thermodynamics Class 11/ Part 22/ Hess's law of constant heat summation
The Hess's law can also be stated as the enthalpy change for a chemical reaction is the same regardless of the path by which the reaction occurs. For example, consider following two paths for the preparation of methylene chloride Path I : CH 4(g)+2Cl2(g) → CH 2Cl2(g)+2H Cl(g) ΔH 0 1 = −202.3kJ Path II :

Hess Law of Constant Heat Summation Chemical Thermodynamics
Understanding Hess Law. Hess's Law, otherwise known as the law of constant heat summation, is a fundamental principle in the field of chemistry. This law asserts that the total enthalpy change (ΔHrec) in a chemical reaction remains constant, regardless of the reaction pathway taken, provided the temperature remains constant.

Hess Law of Heat Summation
Hess's Law of Constant Heat Summation states that r, the total enthalpy change for the reaction is the sum of all changes and does not depend whether it takes place in single or multiple steps. This law is aan outcome of the fact that enthalpy is a state function. The applications of Hess's law are in . Calculation of enthalpy of formation